Abstract
Introduction
Development of anti-CD38 monoclonal antibody therapies (MoABs), including daratumumab and isatuximab, have drastically changed the therapeutic landscape for management of relapsed and/or refractory multiple myeloma (RRMM). However, there is a paucity of information regarding response to treatment after progression on CD38 MoABs. This collaborative research study (MAMMOTH: Monoclonal Antibodies in Multiple Myeloma: Outcomes after Therapy Failure) investigates the therapeutic choices and outcomes of subsequent treatments after refractoriness to MoABs.
Methods
Patients from 14 US academic institutions with diagnosis of MM and refractory to daratumumab or isatuximab, administered alone or in combination, were evaluated. Patients were considered refractory to a CD38 MoAB if treated with at least 4 weeks of therapy and had evidence of progressive disease (PD) while on therapy or within 60 days after last dose. Time of progression was defined as time zero (T0). Data was collected by electronic platform and submitted to peer-based quality check for completeness and internal consistency.
Results
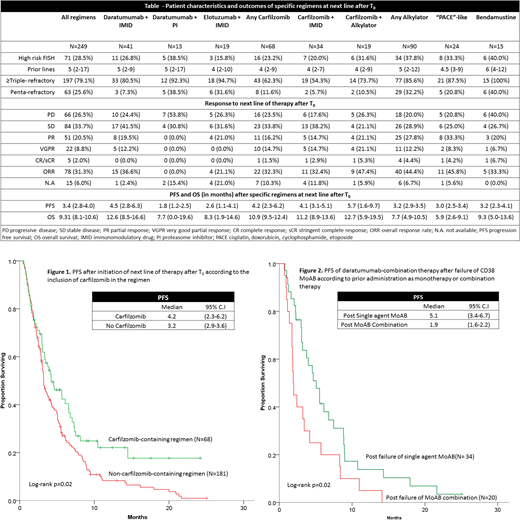
Two hundred and seventy-five patients with MM refractory to CD38 MoAB were evaluated; 249 (90.5%) of those patients who received at least one subsequent line of therapy were included in this analysis. The median age at T0 was 65 years (range 27-90) and 54% were male. At the time of diagnosis, 28% had ISS stage III disease and 29% had high-risk cytogenetics. Patients were heavily pre-treated at T0 with a median of 5 lines of therapy (range 2-17); most (74%) underwent a prior autologous stem cell transplant. The majority of patients were refractory to other anti-myeloma therapies including lenalidomide (78%), pomalidomide (65%), bortezomib (69%) and carfilzomib (45%). In total, 97.1% of patients were exposed to at least one immunomodulatory agent (IMiD) and one proteasome inhibitor (PI), 79.3% were ≥ triple-refractory (refractory to 1 IMiD, 1 PI and 1 CD38 MoAB), while 25.3% were penta-refractory (refractory to 2 IMiDs, 2 PIs and 1 CD38 MoAB). Responses to the next subsequent line of therapy after progression with CD38 MoAB are shown (Table). The overall response rate (ORR; ≥PR) of first regimen post-T0 was 31% with a median progression free survival (PFS) of 3.4 mo and median overall survival (OS) of 9.3 mo. Carfilzomib-based therapy resulted in an ORR of 32% with median PFS 4.2 mo and overall OS of 10.9 mo. The addition of an IMiD to daratumumab yielded an ORR of 37% with median PFS 4.5 mo and OS 12.6 mo. The addition of a PI to daratumumab yielded no responses. Elotuzumab-based therapy had an ORR of 21% with median PFS 2.6 mo and OS 8.3 mo.
By multivariate analysis, alkylator-based therapies were found to have the highest ORR (OR 3.1, 95% CI 1.8-5.7, p<0.001), however, resulted in median PFS of only 3 mo and OS of 7.7 mo. Multivariate analysis evaluating for PFS and adjusting for refractoriness to prior therapies found lower risk of progression with carfilzomib-based therapy (HR 0.60, 95% CI 0.42-0.85, p=0.004, Figure 1) and with the addition of an IMiD to daratumumab (HR 0.64, 95% CI 0.43-0.94, p=0.02). The benefit of IMiD + daratumumab was essentially restricted to patients previously refractory to CD38 MoAB as monotherapy. These patients demonstrated improved PFS with subsequent daratumumab-combination therapy at time of progression compared with patients refractory to CD38 MoAB combination therapy (log-rank p=0.02, Figure 2). No particular regimen was associated with improved OS compared with the rest.
Conclusions
After progression on CD38 MoAB, treatment with carfilzomib-based regimens and the addition of an IMiD to daratumumab-monotherapy resulted in a higher ORR and PFS compared with other regimens. Alkylating agents resulted in the highest ORR but relatively short PFS and OS. The use of elotuzumab and the addition of a PI to daratumumab monotherapy resulted in relatively low response rates. Overall, progression after CD38 MoAB therapy is associated with a poor prognosis and innovative therapeutic strategies are needed.
Malek:Celgene: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau. Paul:Bristol Myer Squibb: Other: Stock and pension plan (past employee). Neppalli:Celgene: Consultancy; Amgen: Consultancy. Liedtke:Amgen/Onyx: Consultancy, Honoraria, Research Funding; Caelum: Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prothena: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; celgene: Research Funding; BlueBirdBio: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hari:Takeda: Consultancy, Honoraria, Research Funding; Spectrum: Consultancy, Research Funding; Kite Pharma: Consultancy, Honoraria; Sanofi: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amgen Inc.: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Janssen: Honoraria. Vij:Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jansson: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Usmani:Amgen, BMS, Celgene, Janssen, Merck, Pharmacyclics,Sanofi, Seattle Genetics, Takeda: Research Funding; Abbvie, Amgen, Celgene, Genmab, Merck, MundiPharma, Janssen, Seattle Genetics: Consultancy. Costa:Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; BMS: Research Funding; Abbvie: Research Funding; Janssen: Research Funding; Karyopharm: Research Funding; Sanofi: Honoraria. Kumar:Roche: Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal